New MDCG guidance on temporary extraordinary measures related to medical device Notified Body audits during COVID-19 quarantine orders and travel restrictions | medicaldeviceslegal
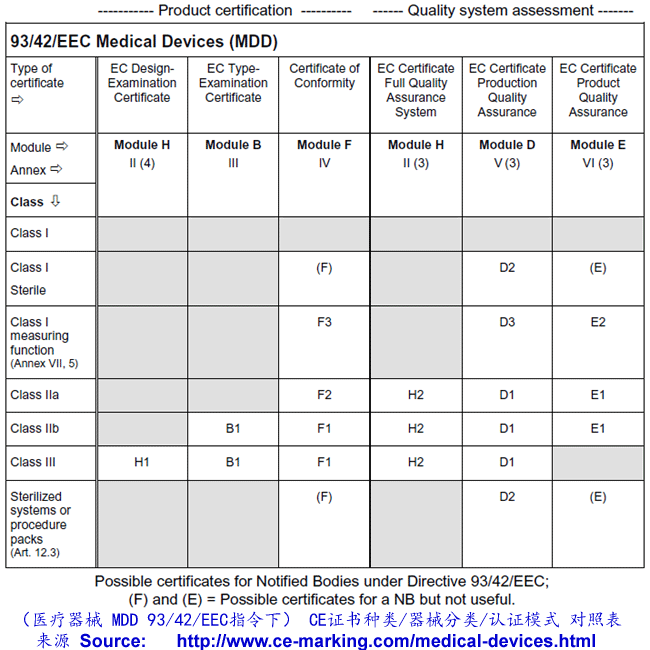
Guide on Class IIb MDD- Medical Devices CE marking (mark) & European (EU) Authorized Representative service
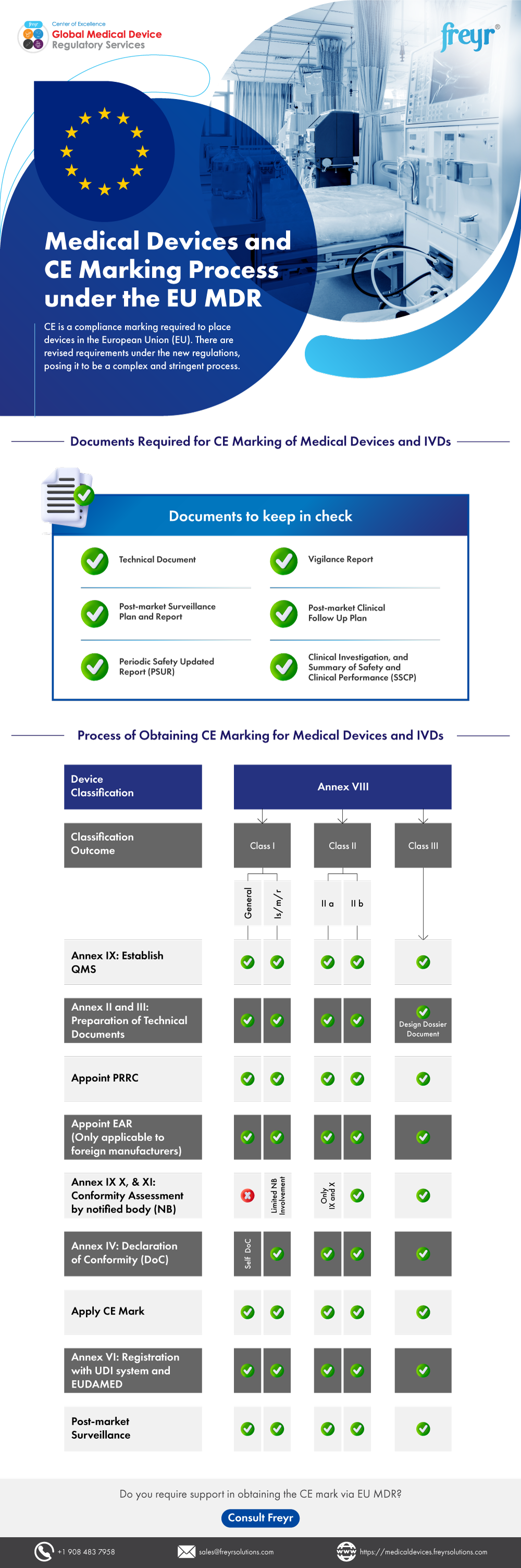
Medical Devices and CE Marking Process under the EU MDR | Freyr - Global Regulatory Solutions and Services Company
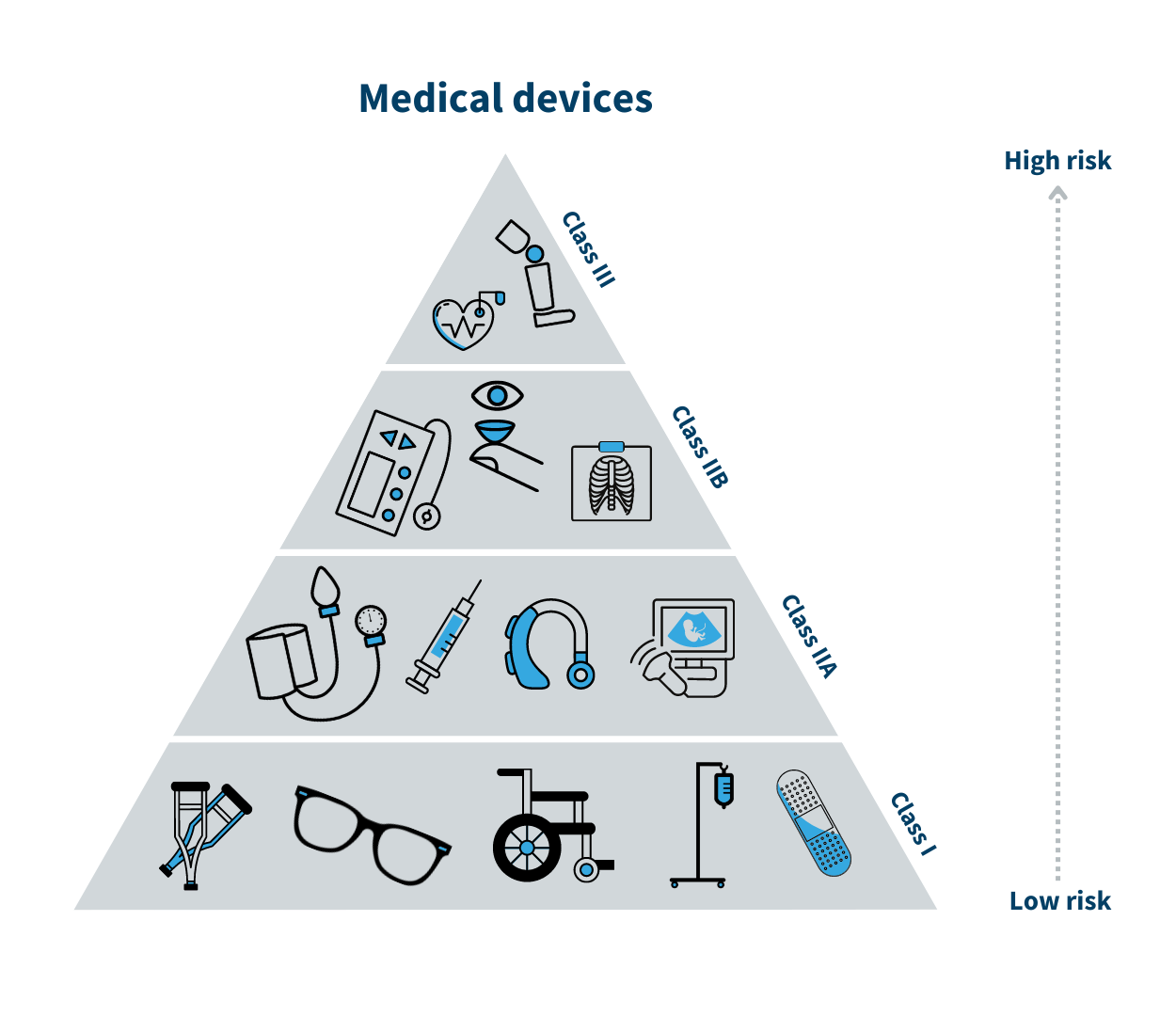


![EU MDR Update: How To Get a New Medical Device Certified? [Flow Charts] - Sofeast EU MDR Update: How To Get a New Medical Device Certified? [Flow Charts] - Sofeast](https://x6t6s6a2.rocketcdn.me/wp-content/uploads/2021/08/class-IIa-medical-devices.jpeg)
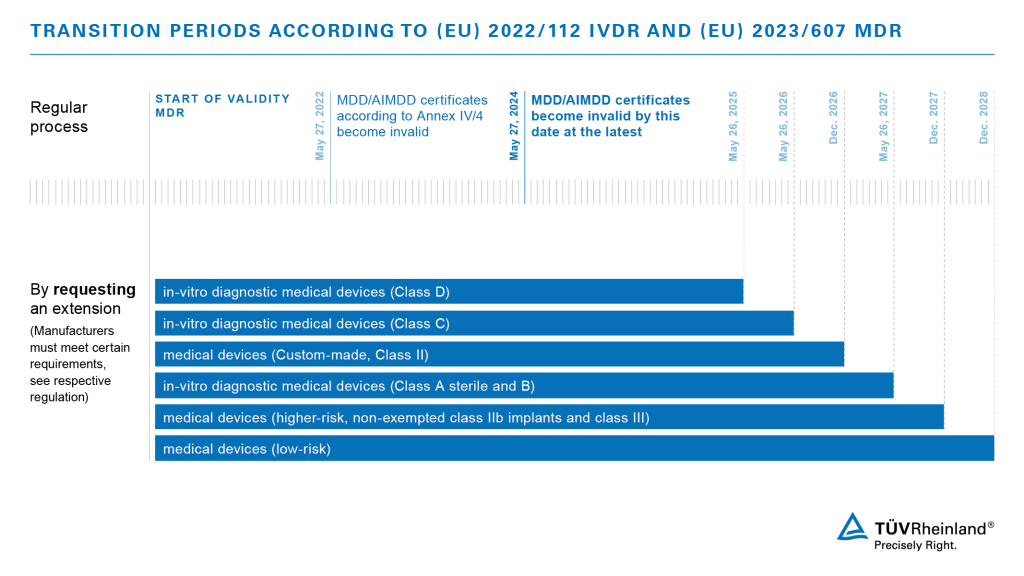
/tuv-rheinland-ivdr-visual-2-en_core_1_x.png)







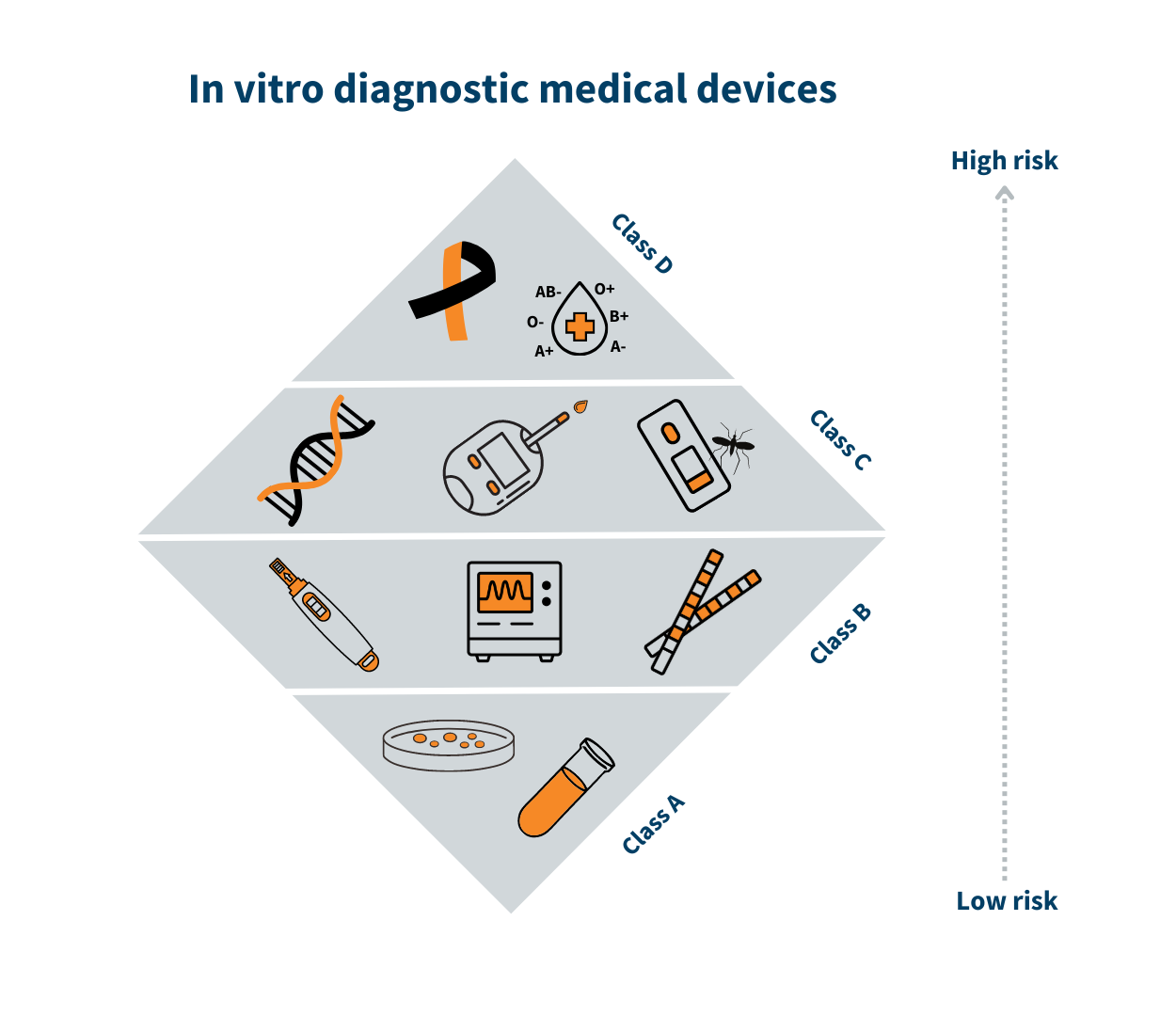
![EU MDR Update: How To Get a New Medical Device Certified? [Flow Charts] - Sofeast EU MDR Update: How To Get a New Medical Device Certified? [Flow Charts] - Sofeast](https://x6t6s6a2.rocketcdn.me/wp-content/uploads/2021/08/class-I-medical-devices.jpeg)